Have you ever wondered how a contaminant with a boiling point above 100°C can be volatilized and removed at temperatures near the boiling point of water? For example, one of the most common chlorinated VOCs, tetrachloroethylene (PCE), has a pure phase boiling point of 121°C, yet more than half of in situ thermal remediation projects treat chlorinated solvent mixtures, many of which include PCE, by targeting the boiling point of water or 100°C ( https://doi.org/10.1111/gwmr.12424).
What is a Volatile Organic Compound (VOC)?
Let’s start with the definition of a VOC. Setting aside the various regulatory definitions, the Environmental Protection Agency describes VOCs as “compounds that have a high vapor pressure and low water solubility”. Other organizations have described a VOC by its boiling point at atmospheric pressure, for example, having an initial boiling point less than or equal to 250°C. Another practical method of defining a VOC is referencing a laboratory’s standard parameter list for VOC analyses, e.g., EPA Method 8260. Note, compounds like naphthalene can be analyzed as both a VOC and a semi-volatile organic compound (SVOC) and define the upper limit of a traditional VOC.
In the context of thermal remediation, the definition of a VOC is also a sliding scale, although coincidently, naphthalene also serves as common transition point between the world of VOCs and SVOCs. For thermal treatment applications, a more apt question is, what compounds can be effectively recovered by heating a site to temperatures near the boiling point of water?
First, let’s cover the primary recovery mechanisms for thermally treating VOC sites, and then we’ll get back to this question.
What are the Thermal Remediation Mechanisms?
There are a few key concepts to keep in mind when understanding how contaminants, including VOCs, are mobilized, and recovered during thermal remediation. When heat is added to the subsurface, the vapor pressure of the contaminant or contaminant mixture increases, exponentially, as shown below. The higher the vapor pressure, the higher the volatility, and when the vapor pressure exceeds the atmospheric pressure, boiling occurs (USACE Design Guide for In Situ Thermal Remediation). These vapors push and find their way through the soil pores below the water table, ultimately reaching the vadose zone where they are then swept to extraction points for recovery. Based on our experience treating VOC sites, a rule of thumb is that 98 percent of the mass present is recovered in the vapor phase, so we know that this is a primary mechanism for mass recovery. A compound’s solubility, or its affinity for dissolving into water, can impact the relative ease at which the compound can be coaxed into the vapor phase and recovered. A classic example is 1,4-dioxane, which has a pure phase boiling point of 101°C but is highly soluble in water or miscible which makes it more difficult to volatilize and recover than compounds with similar boiling points.
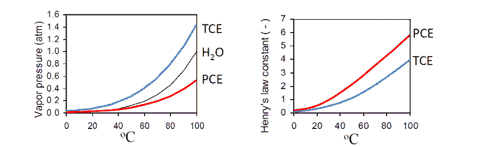
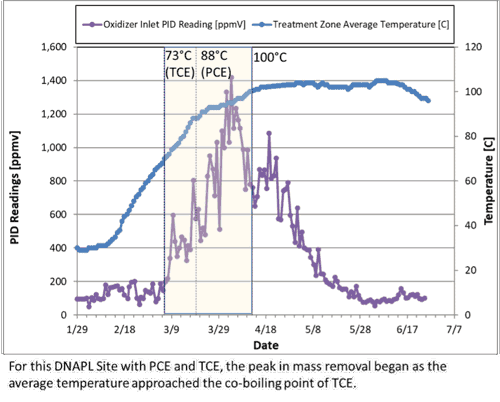
For sites with contaminants in the non-aqueous phase or present as a NAPL, the boiling point of a liquid mixture (i.e., PCE and water) is different than the pure phase boiling point. Without going into the details of summing partial pressures, know that this phenomenon can effectively lower the temperature at which volatilization occurs. Continuing with our example, for a mixture of PCE (BP = 121°C) and water (BP = 100°C), the co-boiling point is 88.5°C. You may have heard other terms like eutectic boiling point, azeotropic boiling point. As a real-world check, when thermally treating source zones with NAPL, the peak of mass recovery often corresponds to the co-boiling point of a mixture of water and the dominant site COCs.
Lastly, the concept of steam stripping or steam distillation cannot be ignored. A key thermal design parameter revolves around injecting enough energy to transform a certain percentage of water in the pore spaces to steam. That percentage depends on multiple factors – more on that later. As that steam is generated in situ, it strips contaminants tied up in the treatment zone and carries them to an extraction point. Steam stripping is a process used in the wastewater treatment and the petroleum industry to remove or separate VOCs from liquid mixtures, so it’s not a surprise that steam stripping is effective for recovering VOCs in situ.
There are other contaminant removal mechanisms that might be applicable, like in situ degradation through hydrolysis, or physical recovery at NAPL-heavy sites, but the primary removal mechanisms at most VOC sites are volatilization and steam stripping.
Back to that question…
What compounds can be effectively recovered at temperatures near the boiling point of water?
Below is a simplified list of compounds grouped into categories that describes how easily they are recovered at boiling temperatures. This is an empirical list based on our experience treating complex mixtures of contaminants in various geologies. However, this helps describe how temperature affects vapor pressure and how solubility combined with a compound’s pure phase boiling point can be an indicator of its recoverability. From a design standpoint, the easier VOCs may require less steam stripping or a lower percentage of pore volume boil-off compared to more challenging VOCs.

In practice, easier to medium range VOCs may require boiling off 20 to 30% of the estimated pore volume. For more stringent cleanup goals, soils with higher total organic carbon content (which VOCs tend to cling to), and more challenging VOCs, up to 50% boiloff may be required. For miscible compounds like 1,4-dioxane, 50-70% boiloff may be required. For more information on how much energy is required for treating VOCs and SVOCs based on our project database, checkout our blog, Have you Ever Wondered How Much Energy it Takes?
Thanks for taking the time to read this. If you have any questions on how thermal remediation works or if the chemicals and mixtures at your site can be treated with thermal and what the design and costs would look like, please contact me.
If you’re considering ISTR technology or simply want to learn more, register for our webinar, How are VOCs Treated using Thermal Remediation?, or contact our team of thermal remediation experts.
04.06.23
Erin Hauber
Ms. Erin Hauber has over 15 years of experience as a remediation engineer in the environmental industry, focusing on development of remedial treatment strategies with an emphasis on in situ thermal remediation and injection-based technologies. As Senior Techno...


